Six children, born deaf, received a revolutionary gene therapy treatment through an ear injection, resulting in five of them regaining their hearing. The experimental approach shows promise for potential applications in other medical treatments.
The study, conducted in Fudan, China, was co-led by researchers from Harvard Medical School at the Massachusetts Eye and Ear. Six children, aged one to seven, with inherited deafness due to OTOF gene mutation, were treated. The OTOF gene is responsible for producing a crucial protein involved in transmitting signals from the ear to the brain.
During the 26-week trial, five out of the six children displayed signs of improved hearing, with four outcomes described as “robust,” as per Harvard Medical School. The study results, published in the journal Lancet, highlighted the critical role of hearing ability in language learning. The researchers also measured speech perception, and all five responsive children showed improvement in recognizing sounds as speech.
Co-senior author Zheng-Yi Chen, an associate professor of otolaryngology, head and neck surgery at Harvard Medical School and a researcher at Mass Eye and Ear’s Eaton-Peabody Laboratories, stated, “This really opens the door to developing other treatments for different kinds of genetic deafness.”
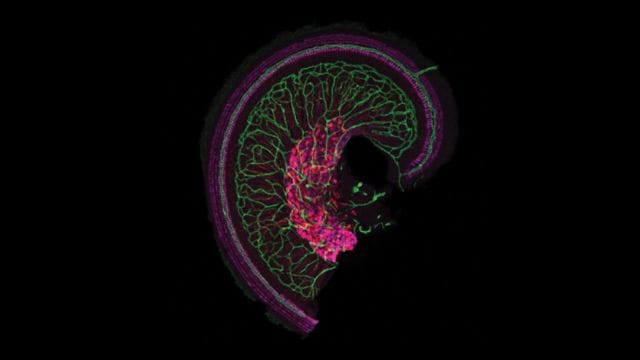
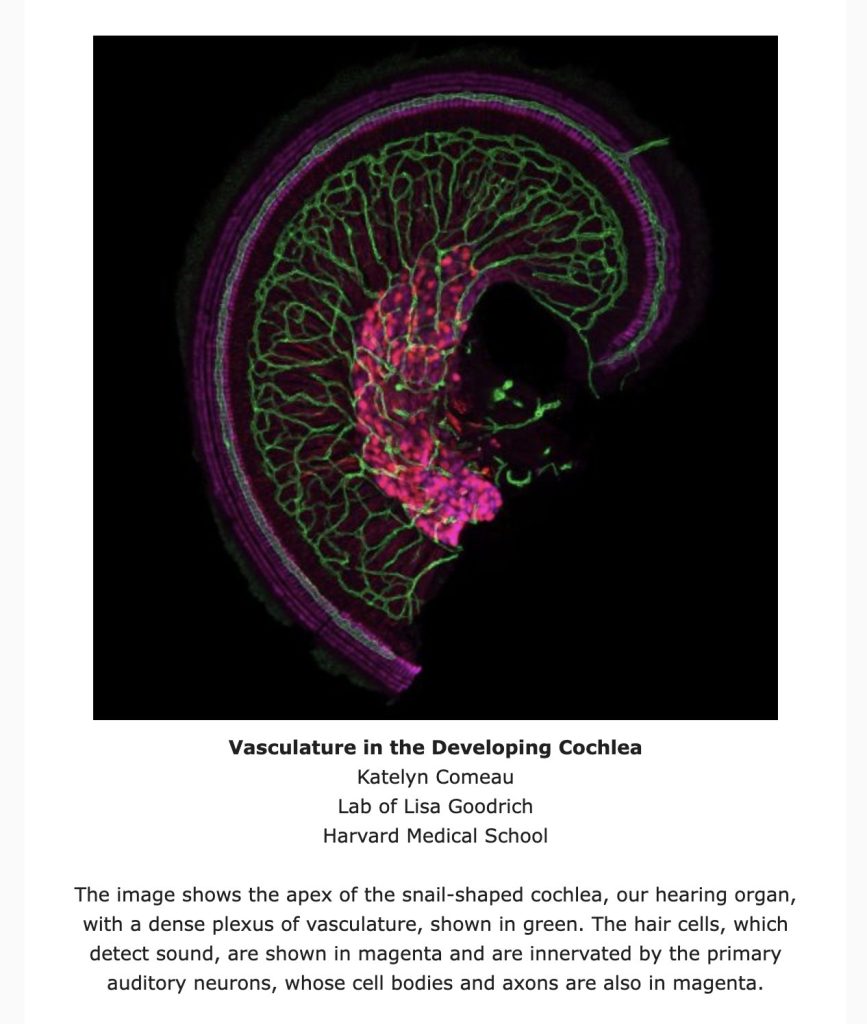
The OTOF gene is responsible for producing the otoferlin protein, a crucial component produced by cells in the cochlea, the snail-shaped part of the inner ear. The cochlea translates sound waves into electric pulses, which are then conveyed to the brain by nerve cells. Otoferlin plays a vital role in transmitting pulses from cochlear cells to nerves. Without it, although sound would be translated into electric signals, they would fail to reach the brain.
The OTOF gene mutation present in the children is an appealing target for gene therapy due to its simplicity—it results from a single mutation. Moreover, it doesn’t entail any physical damage to the cochlear cells.
When the researchers sought study participants, they garnered a substantial response of 425 individuals, underscoring the demand for enhanced treatments for congenital deafness lacking approved drugs. Following screening, only six participants were eventually enrolled.
Of the six, four children had cochlear implants, which, with training, enabled the comprehension of speech and sound. The two youngest participants, aged one and two, had no implants. When the implants were deactivated, all participants experienced complete deafness.
The researchers employed a method wherein the gene was introduced into the cochlea using a specific virus commonly utilized for such treatments. However, a challenge arose—the OTOF gene’s size exceeded the virus’s capacity.
To overcome this hurdle, the researchers divided the gene into two parts and enclosed each half in distinct viruses. They then injected this mixture into the cochlea. Despite the viruses incorporating the gene halves into different segments of the cells’ DNA, the cellular machinery assembled the complete protein upon expression.
This unique mixture was injected into the inner ear fluid, and the viruses navigated to the target cells in a manner typical of a standard injection. Both the researchers and participants awaited the initial signs of restored hearing for four to six weeks in each case.
Five of the six participants exhibited indications of progressive improvement. By the 26th week, when the cochlear implants were deactivated for the three older children, they could comprehend and respond to speech. Two of them even demonstrated the ability to recognize speech in a noisy environment and engage in a telephone conversation. The younger children also displayed improvements, though they were too young for certain tests.
Notably, one participant showed no signs of improvement. While the exact cause remains uncertain, the researchers speculate it may be attributed to an immune response to the viral vector.